Practice Drawing Lewis Structures Organic Chemistry
1.E: Structure and Bonding in Organic Molecules (Exercises)
-
- Last updated
- Save as PDF
- Page ID
- 54803
These are homework exercises to accompany Chapter 1 of Vollhardt and Schore's "Organic Chemistry" Textmap.
1.8: Hybrid Orbitals: Bonding in Complex Molecules and Practice Problems
Q25
Draw the Lewis Structures of the following molecules, and if necessary assign charges.
- BrF
- ClCN
- SOCl2
- CH3CH2NH2
- CH3CH2OCH2CH3
- (CH3)2NNH2
- CH3NCNCH3
- CH3COOH
- N2O4
Q26
In problem 25 identify the polar covalent bonds with δ+ and δ- on the atoms according to their electronegativity.
Q27
Draw the Lewis Structures of the following species, and if necessary assign charges.
- H3O+
- NH4 +
- CH3CH2 -
- CH3CH2 +
- CH3CH2
- CH3CH
- H-
- CH3COO-
Q28
Add charges to the following Lewis Structures, if neutral do not add any charges.
(a) 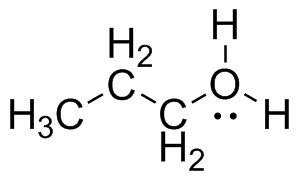 (b)
(b)  (c)
(c) 
(d) 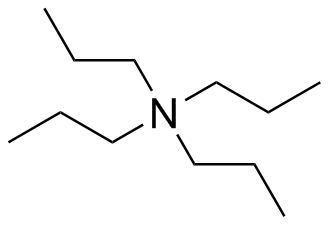 (e)
(e) 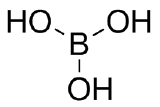 (f)
(f)
Q29
Sulfuric Acid is a known strong acid, it's deprotonated form SO4 2-, has several resonance structures. Draw the remaining two using electron pushing arrows.

Acetic acid is a common household acid once deprotonated it forms an anion with resonance structures possible. Draw these with electron-pushing arrows.

Q30
In problem 25 several of the species have possible resonance structures. Draw the electron-pushing arrows and resulting resonance structures for 25.c, 25.h, 25.i. Identify the major contributor, and give reasons.
Q31
Draw all possible resonance structures of the following species.
- CH2COCH3 -
- CH2N2
- CO
- CH2ON(CH3)2
- SCN-
- NO3 2-
- O3
Q32
Draw all possible resonance structures of the following. Based on the resonance structures, which carbonyl carbon, marked in red, would be the most electron deficient?
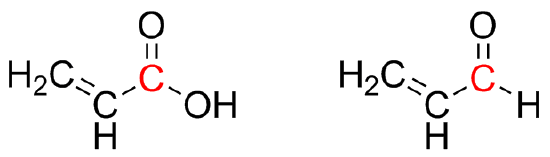
Q33
Draw the Lewis structure for each compound.
- Fluorine and the Fluoride anion
- GeH4 and GeO
- H2O2 (Hydrogen Peroxide)
- Phosphine (PH3) and Ammonia (NH3)
Q34
Draw the molecular orbital diagrams of H2 +, He2 2+, and He2, and indicate the bond order. Compare and contrast the molecular orbital diagrams of He2 + and He2, which is more favorable?
Q35
Predict the geometry for each red-highlighted atom and give the hybridization of that center.
(a)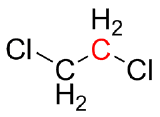 (b)
(b)  (c)
(c)  (d)
(d)  (e)
(e)  (f)
(f) 
Q36
In problem 35 indicate the hybrid orbitals' interactions (sp3, sp2, sp) and the atomic orbitals used (s, p).
Q37
Draw the hybridized orbitals for each of the highlighted carbons in problem 35.
Q38
Determine the hybridization of each carbon based on the shape of each molecule.
(a) CH3Br (b) CH3CH2OH (c) H3CHCCH (d) CH3 - (e) NH4 +
Q39
Draw the Kekule structures for the following molecules, an example is given below.
Example. CH3CH2CH2CH3

(a) CH3CH2OH (b) (CH3)2CH2OH (c) (CH3)2NCHO (d) CH2ClCH2Cl (e) HOOCCH2COOH
Q40
Draw the Kekule structures for the following molecules.
(a)  (b)
(b)  (c)
(c)  (d)
(d)  (e)
(e)  (f)
(f) 
Q41
Convert the following structures into condensed formulas
(a) 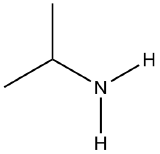 (b)
(b)  (c)
(c) 
Q42
Give the condensed structures for the following Kekulé structures.
(a)  (b)
(b)  (c)
(c) 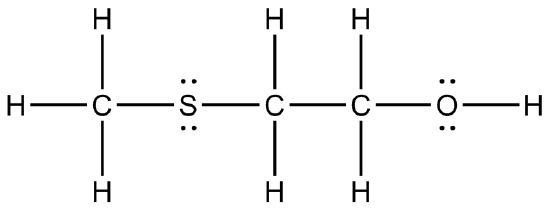 (d)
(d)  (e)
(e)  (f)
(f) 
Q43
In the previous problem, 42, draw the Kekulé structures as bond line structures.
Q44
Draw the following condensed formulas as hashed-wedged line structures.
- CH3CH2OCH2CN
- CH2Cl2
- (CH3CH2)3P
- CH2CHCH2SiH3
Q45
Draw all possible constitutional isomers, in bond-line form, ofIthe following molecules.
(a) C3H8 (b) C6H14 (c) C4H10
Q46
Draw line-bond structures of the following pairs of species and include all charges. Are any of the pairs resonance forms of the other species in the pair? If not explain the difference
(a) H3CCHCCH2 and H2CCCHCH3 (b) HCN and H3CNH2 (c) CH3CH2COH and CH3CH2CHOH
Q47
The following boron-halogen compounds have resonance structures possible. Draw them and in each compound indicate which structure is favored (hint: the octet rule takes precedent). Also, compare the relative energy levels of the three favored resonance structures, which is favored/lowest in energy?
(a) (CH3)2BF (b) (CH3)2BCl (c) (CH3)2BBr
Q48
Using the formula for the degrees of each angle, [(n-2)*180]/n, where n equals the number of sides, determine the angles in each of the following shapes. If the given shapes were considered bond-line structures, order them from highest to lowest favorability based on the angles (The best overlap in sp3 hybridized carbon is at 104.5o). Compare this answer to the following ring strain energies of each: cyclopropane (triangle)- 27.5 kcal/mol cyclobutane (square)- 26.3 kcal/mol cyclopentane (pentagon)- 6.2 kcal/mol cyclohexane (hexagon)-0.1 kcal/mol.

Q49
Give the hybridization orbital that the lone pair of each of the following species belongs in. Also, given that the 2p orbitals are higher in energy than the 2s orbitals, order the species from lowest to highest energy according to the s/p character. (sp3= 1/4s and 3/4p) (sp2= 1/3s and 2/3p) (sp= 1/2s and 1/2p).
(a) CH3CH2CH2 - (b) CH3CHCH- (c) CH3CC-
Based on their relative energy, which species would it be most favorable to undergo the following dissociation (HA represents the neutral hydrocarbon)?

Q50
In each set which of the following positively polarized carbon bond atoms is the most electropositive?
(a) (i)CHCl3 (ii) CHCl2 (iii) CCl4
(b) (i) ClCH2OCH2Cl (ii) CH3OCH2Cl
(c) (i) (CH3)3C+ (ii) (CH3CH2)3C+
Q51
The following molecule is a common insecticide, cyfluthrin. It is known to be very effective against invertibrates, but it is not nearly as toxic to mammals. In the following highlight one (a) highly polarized covalent single bond (b) one highly polarized double bond (c) one sp2 hybridized carbon atom (d) one sp hybridized carbon atom and (e) one conjugated system.

Q52
In the following reaction does the hybridization of the highlighted carbon atom change? Give the hybridization before and after.

Q53
A combustion elemental analysis finds that a compound is made up of 84% carbon and 16% hydrogen (C=12.0, H=1.0, O=16.0). Which of the following could be the compound tested?
(a) CH4O (b) C6H14O2 (c) C14H22 (d) C7H16
Q54
As drawn, which of the following is a correct formal charge assignment?

(a) +1 on F (b) -1 on N (c) +2 on N (d) -1 on B (e) +1 on B
Q55
The highlighted bond is formed by the interaction between what two orbitals?

Q56
Which highlighted carbon center has the smallest bond angles?
(a) CO2 (b) CH2Cl2 (c) HCCH (d) CO
Q57
Which pair of structures are resonance hybrids?
(a)  (b)
(b)  (c)
(c)  (d)
(d) 
Solutions
S25.
(a) (b)
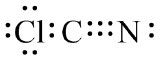 (c)
(c) 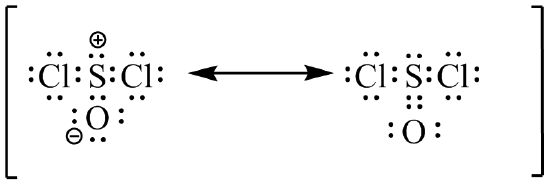 (d)
(d) 
(e) (f)
(f) 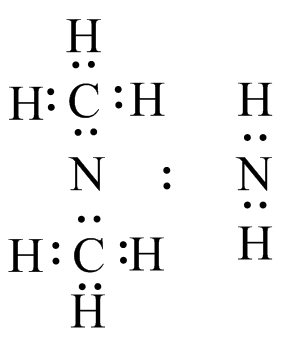 The lone pair is shared between the two N atoms.
The lone pair is shared between the two N atoms.
(g)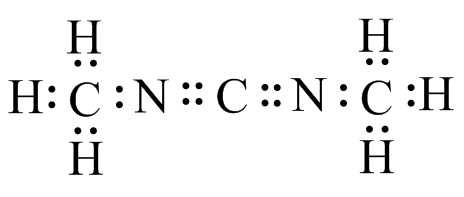 (h)
(h)  (i)
(i) 
S26
(a)  (b)
(b)  (c)
(c) 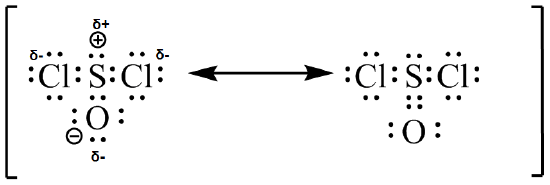 (d)
(d) 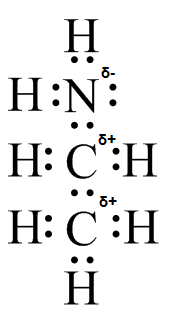
(e)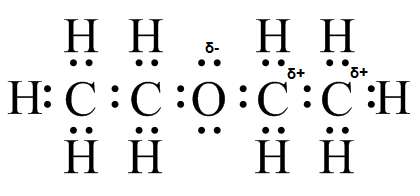 (f)
(f)  (g)
(g) 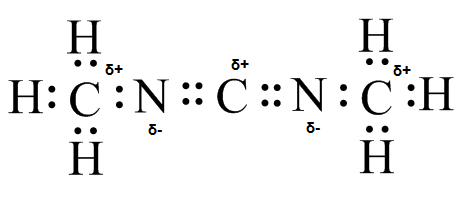
(h)  (i)
(i) 
S27
(a)  (b)
(b)  (c)
(c) 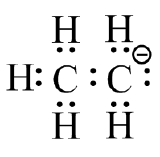 (d)
(d) 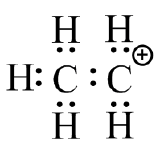
(e) 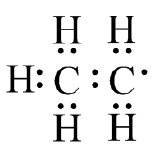 (f)
(f)  (g)
(g)  (h)
(h) 
S28
(a)  (b)
(b) (c)
(c) 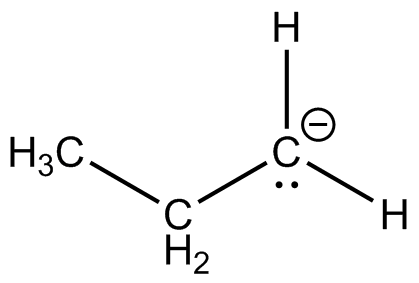
(d)  (e)
(e)  neutral (f)
neutral (f) 
S29
Sulfuric acid has two more possible resonance structures. Later, resonance/charge distribution will be mentioned; the resonance structures possible for the deprotonated sulfuric acid are one reason for why it is so strongly acidic.

Acetic acid has one additional resonance structure possible, again a reason for its moderate acidity.

S30
(c)  The resonance structure with no charges is the major contributor.
The resonance structure with no charges is the major contributor.
(h)  Again, the resonance structure with no charges is the major contributor.
Again, the resonance structure with no charges is the major contributor.
(i)  Each resonance structure is equivalent, so there is no "major contributor".
Each resonance structure is equivalent, so there is no "major contributor".
S31
(a)  (b)
(b) 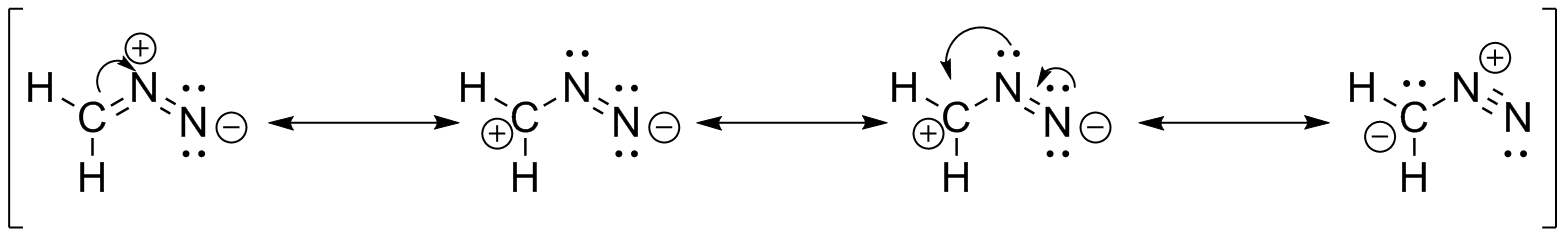
(c)  (d)
(d) 
(e)  (f)
(f) 
(g) 
S32
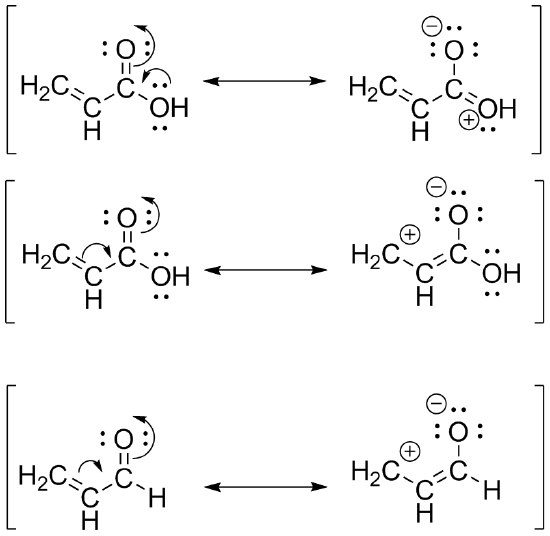
The first structure has two possible resonance structures, further distributing electrons more among the carbonyl carbon. However, the second structure only has one possible resonance structure. This distributes electrons to a lesser extent among the carbonyl carbon. Thus, in the second structure, the aldehyde, the carbon marked in red is more electron deficient than in the carboxylic acid.
S34

H2 + has a bond order of 1/2, He2 + has a bond order of 1/2, and He2 has a bond order of 0. The zero bond order indicates that He2 is not favorable.
S35
- dichloroethane: The highlighted carbon center is tetrahedral with a hybridization of sp3; it is bonded to four other groups.
- acetic acid: The carbon center is trigonal planar with a hybridization of sp2; it is bonded to three groups and has no lone pairs.
- propene: The carbon center is trigonal planar with a hybridization sp2; it is bonded to three groups and has no lone pairs.
- acetylene: The carbon center is linear with a hybridization of sp; it is bonded to two groups with no lone pairs.
- triethylamine: The nitrogen center is tetrahedral with a hybridization of sp3; it is bonded to three groups with one lone pair.
- triethylammonium ion: The nitrogen center is tetrahedral with a hybridization of sp3; it is bonded to four groups with no lone pairs. All are sigma bonds.
S36
- The highlighted carbon forms bonds with one chlorine, two hydrogen atoms, and one other carbon. Each of these atoms connect to the central carbon forming sp3-sp3 hybridized orbitals. The carbon and hydrogen both use electrons from the s orbital, while the bonding electrons from the chlorine are from the p orbitals. All are sigma bonds.
- The central carbon is sp2 hybridized, and it is bonded to one carbon and two oxygen atoms. The bond to the carbon involves an sp3-sp2 overlap with the s orbital on the adjacent carbon involved in bonding, sigma bonding. The carbon oxygen double bond is slightly more complicated. The oxygen is less likely to undergo orbital hybridization. So, the C(sp2)-O(p) sigma bond is formed along with the interaction of the second oxygen p orbital with the carbon p orbital to yield a pi bond. The carbon oxygen bond is similar forming a sigma bond through the C(sp2)-O(p) interaction.
- The central carbon is sp2 hybridized and bonded to two other carbons and one hydrogen atom. The bond between the hydrogen atom involves the C(sp2)-H(s) interaction yielding a sigma bond. The C=C double bond involves the C(sp2)-C(sp2) interaction creating a pi bond. The C-C single bond is a sigma bond involving the C(sp2)-C(sp3) hybrid orbitals.
- The highlighted carbon is bonded to one hydrogen and one carbon atom. The C-H single bond is made up of the C(sp)-H(s) interaction yielding a sigma bond. The C-C triple bond is made up of the C(sp)-C(sp) hybrid orbitals yielding a sigma bond, s orbitals, and two pi bonds, using the two p orbitals.
- The highlighted nitrogen is bonded to three identical carbon groups and has one lone pair. Each of the carbon atoms is sp3 hybridized. So, a N(sp3)-C(sp3) yields a sigma bond.
- The highlighted nitrogen is bonded to three identical carbon atoms and one hydrogen. There are no lone pairs on the nitrogen, and it is sp3 hybridized. The N-C bonds are the same as in part (e), N(sp3)-C(sp3) yielding a sigma bond. The N-H bond is formed from the N(sp3)-H(s) interaction, also a sigma bond.
S37
(a)  (b)
(b)  (c)
(c) 
(d) 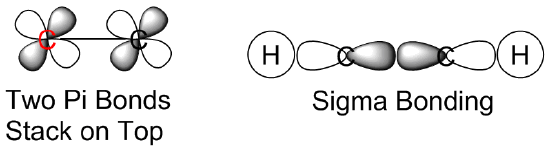 (e)
(e)  (f)
(f) 
S38
Each carbon's hybridization is listed from left to right in the chemical formula.
- tetrahedral, sp3
- tetrahedral, sp3 ; tetrahedral, sp3
- tetrahedral, sp3 ; linear, sp ; linear, sp
- tetrahedral, sp3
- tetrahedral, sp3
S40
(a) (b)
(b)  (c)
(c)  (d)
(d) 
(e)  (f)
(f) 
S41
(a) (CH3)2CHNH2 (b) CClH2CH2Cl (c) CH3I
S42
(a) CH3CH2NH2 (b) CH3 C=O CH3 (c) CH3SCH2C2OH (d) CF3CHOHCF3 (e) CH2CCH2 (f) CH2CH C=O OCH3
S46
(a)  the two structures are identical (b)
the two structures are identical (b)  These are not resonance structures; there additional H atoms. (c)
These are not resonance structures; there additional H atoms. (c)  These are not resonance structures; there are three additional H atoms.
These are not resonance structures; there are three additional H atoms.
S47
 In each case the double bonded resonance structure is preferred, due to the octet rule being fulfilled.
In each case the double bonded resonance structure is preferred, due to the octet rule being fulfilled.
The lowest energy structure can be determined by thinking about the electronegativity of each halogen atom. The higher the electronegativity, the less favorable a positive charge is. The order of the electronegativity is as follows, F>Cl>Br, and so the reverse is the order of favorability. (CH3)2BF (b) (CH3)2BCl (c) (CH3)2BBr
S48
Triangle- 60o Square- 90o Pentagon- 108o Hexagon- 120o
This would lead to the expectation that the order of favorability is Pentagon>Square>Hexagon>Triangle. However, the order differs and is in fact Hexagon>Pentagon>Square>Triangle.
In reality this is due to different conformations of the shapes, so that the hexagon and pentagon are no longer flat/planar. This will be covered in more detail later on.
S49
CH3CH2CH2 - - sp3 CH3CHCH- - sp2 CH3CC- - sp
The order of energy is as follows, or increasing p character. CH3CC-<CH3CHCH-<CH3CH2CH2 -
The reaction would be most favorable if the A- is lowest in energy, so, the order of favorability is the reverse of above. CH3CH2CH2 -<CH3CHCH-<CH3CC-
S50
(a) CCl4
(b) ClCH2OCH2Cl
(c) (CH3)3C+ The additional hydrocarbons on the ethyl group do marginally hyperconjugate and donate electrons to the center carbon, making it less electropositive.
S51
(a)  In this structure any sp3 carbon connected to a heteroatom would be a polarized bond.
In this structure any sp3 carbon connected to a heteroatom would be a polarized bond.
(b)  In this structure this is the only highly polarized double bond.
In this structure this is the only highly polarized double bond.
(c)  Any double bonded carbon could be considered sp2 hybridized.
Any double bonded carbon could be considered sp2 hybridized.
(d)  This is the only sp hybridized carbon.
This is the only sp hybridized carbon.
(e) These two benzene rings are conjugated system.
These two benzene rings are conjugated system.
S52
The hybridization changes from sp2 to sp3.
S53
(d) 84/12=7 Carbon and 14/1=14 Hydrogen
S54
(d) is correct, boron typically has 3 valence electrons. It currently is bonded to four different atoms; it has an octet. 8/2=4 electrons, and 3-4=-1 formal charge.
S55
The bond is formed by the interaction between the s orbital of the hydrogen and the sp2 orbital of the carbon.
S56
(b) This is the only sp3 hybridized highlighted carbon, characterized by bond angles of 104.5o. Which is less than both sp2 and sp, 120o and 180o respectively.
S57
(c) The number of electrons is the same and all atoms are in the same locations. Placement of charges is important to look at; in the pairs with no charges present then some atoms were moved around.
Practice Drawing Lewis Structures Organic Chemistry
Source: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Exercises%3A_Organic_Chemistry/Exercises%3A_Volhard_and_Schore/1.E%3A_Structure_and_Bonding_in_Organic_Molecules_(Exercises)
Posted by: waterswittionfer93.blogspot.com

0 Response to "Practice Drawing Lewis Structures Organic Chemistry"
Post a Comment